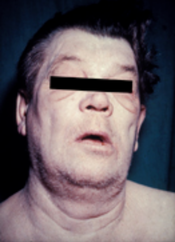
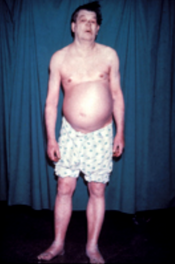
Hypothyroidism
| Hypothyroidism | |
|---|---|
| Other names | Underactive thyroid, low thyroid, hypothyreosis |
 | |
| Molecular structure of thyroxine, which is deficient in hypothyroidism | |
| Pronunciation | |
| Specialty | Endocrinology |
| Symptoms | extreme fatigue, poor ability to tolerate cold, feeling tired, muscle aches, constipation, weight gain,[3] depression, anxiety, irritability[4] |
| Complications | During pregnancy can result in cretinism in the baby[5] |
| Usual onset | > 60 years old[3] |
| Causes | Iodine deficiency, Hashimoto's thyroiditis[3] |
| Diagnostic method | Blood tests (thyroid-stimulating hormone, thyroxine)[3] |
| Differential diagnosis | Depression, dementia, heart failure, chronic fatigue syndrome[6] |
| Prevention | Salt iodization[7] |
| Treatment | Levothyroxine[3] |
| Frequency | 5% (USA)[8] |
Hypothyroidism (also called underactive thyroid, low thyroid or hypothyreosis) is a disorder of the endocrine system in which the thyroid gland does not produce enough thyroid hormones.[3] It can cause a number of symptoms, such as poor ability to tolerate cold, extreme fatigue, muscle aches, constipation, slow heart rate, depression, and weight gain.[3] Occasionally there may be swelling of the front part of the neck due to goitre.[3] Untreated cases of hypothyroidism during pregnancy can lead to delays in growth and intellectual development in the baby or congenital iodine deficiency syndrome.[5]
Worldwide, too little iodine in the diet is the most common cause of hypothyroidism.[8][9] Hashimoto's thyroiditis is the most common cause of hypothyroidism in countries with sufficient dietary iodine.[3] Less common causes include previous treatment with radioactive iodine, injury to the hypothalamus or the anterior pituitary gland, certain medications, a lack of a functioning thyroid at birth, or previous thyroid surgery.[3][10] The diagnosis of hypothyroidism, when suspected, can be confirmed with blood tests measuring thyroid-stimulating hormone (TSH) and thyroxine (T4) levels.[3]
Salt iodization has prevented hypothyroidism in many populations.[7] Thyroid hormone replacement with levothyroxine treats hypothyroidism.[3] Medical professionals adjust the dose according to symptoms and normalization of the thyroxine and TSH levels.[3] Thyroid medication is safe in pregnancy.[3] Although an adequate amount of dietary iodine is important, too much may worsen specific forms of hypothyroidism.[3]
Worldwide about one billion people are estimated to be iodine-deficient; however, it is unknown how often this results in hypothyroidism.[11] In the United States, hypothyroidism occurs in approximately 5% of people.[8] Subclinical hypothyroidism, a milder form of hypothyroidism characterized by normal thyroxine levels and an elevated TSH level, is thought to occur in 4.3–8.5% of people in the United States.[8] Subclinical hypothyroidism has been associated with an increased risk of atrial fibrillation (AF) and is the most frequent thyroid abnormality in acute new-onset AF. [12]Hypothyroidism is more common in women than in men.[3] People over the age of 60 are more commonly affected.[3] Dogs are also known to develop hypothyroidism, as are cats and horses, albeit more rarely.[13] The word hypothyroidism is from Greek hypo- 'reduced', thyreos 'shield', and eidos 'form', where the two latter parts refer to the thyroid gland.[14]
Signs and symptoms
[edit]People with hypothyroidism often have no or only mild symptoms. Numerous symptoms and signs are associated with hypothyroidism and can be related to the underlying cause, or a direct effect of having not enough thyroid hormones.[15][16] Hashimoto's thyroiditis may present with the mass effect of a goitre (enlarged thyroid gland).[15] In middle-aged women, the symptoms may be mistaken for those of menopause.[17]
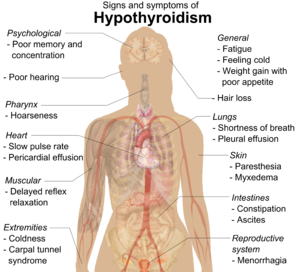
| Symptoms[15] | Signs[15] |
|---|---|
| Fatigue | Dry, coarse skin |
| Feeling cold | Cool extremities |
| Poor memory and concentration | Myxedema (mucopolysaccharide deposits in the skin) |
| Constipation, dyspepsia[18] | Hair loss |
| Weight gain with poor appetite | Slow pulse rate |
| Shortness of breath | Swelling of the limbs |
| Hoarse voice | Delayed relaxation of tendon reflexes |
| In females, heavy menstrual periods (and later light periods) | Carpal tunnel syndrome |
| Abnormal sensation | Pleural effusion, ascites, pericardial effusion |
| Poor hearing | |
| Muscle weakness |
Delayed relaxation after testing the ankle jerk reflex is a characteristic sign of hypothyroidism and is associated with the severity of the hormone deficit.[8]
Myxedema coma
[edit]Myxedema coma is a rare but life-threatening state of extreme hypothyroidism. It may occur in those with established hypothyroidism when they develop an acute illness. Myxedema coma can be the first presentation of hypothyroidism. People with myxedema coma typically have a low body temperature without shivering, confusion, a slow heart rate and reduced breathing effort. There may be physical signs suggestive of hypothyroidism, such as skin changes or enlargement of the tongue.[19]
Pregnancy
[edit]Even mild or subclinical hypothyroidism leads to possible infertility and an increased risk of miscarriage.[20][21] Hypothyroidism in early pregnancy, even with limited or no symptoms, may increase the risk of pre-eclampsia, offspring with lower intelligence,[22][23][24][25] and the risk of infant death around the time of birth.[20][21][26] Women are affected by hypothyroidism in 0.3–0.5% of pregnancies.[26] Subclinical hypothyroidism during pregnancy is associated with gestational diabetes, low birth-weight, placental abruption, and the birth of the baby before 37 weeks of pregnancy.[22][27][28][29]
Children
[edit]Newborn children with hypothyroidism may have normal birth weight and height (although the head may be larger than expected and the posterior fontanelle may be open). Some may have drowsiness, decreased muscle tone, poor weight gain, a hoarse-sounding cry, feeding difficulties, constipation, an enlarged tongue, umbilical hernia, dry skin, a decreased body temperature, and jaundice.[30] A goiter is rare, although it may develop later in children who have a thyroid gland that does not produce functioning thyroid hormone.[30] A goitre may also develop in children growing up in areas with iodine deficiency.[31] Normal growth and development may be delayed, and not treating infants may lead to an intellectual impairment (IQ 6–15 points lower in severe cases). Other problems include the following: difficulty with large scale and fine motor skills and coordination, reduced muscle tone, squinting, decreased attention span, and delayed speaking.[30] Tooth eruption may be delayed.[32]
In older children and adolescents, the symptoms of hypothyroidism may include fatigue, cold intolerance, sleepiness, muscle weakness, constipation, a delay in growth, overweight for height, pallor, coarse and thick skin, increased body hair, irregular menstrual cycles in girls, and delayed puberty. Signs may include delayed relaxation of the ankle reflex and a slow heartbeat.[30] A goiter may be present with a completely enlarged thyroid gland;[30] sometimes only part of the thyroid is enlarged and it can be knobby.[33]
Causes
[edit]Hypothyroidism is caused by inadequate function of the gland itself (primary hypothyroidism), inadequate stimulation by thyroid-stimulating hormone from the pituitary gland (secondary hypothyroidism), or inadequate release of thyrotropin-releasing hormone from the brain's hypothalamus (tertiary hypothyroidism).[8][34] Primary hypothyroidism is about a thousandfold more common than central hypothyroidism.[10] Central hypothyroidism is the name used for secondary and tertiary, since hypothalamus and pituitary gland are at the center of thyroid hormone control.
Iodine deficiency is the most common cause of primary hypothyroidism and endemic goitre worldwide.[8][9] In areas of the world with sufficient dietary iodine, hypothyroidism is most commonly caused by the autoimmune disease Hashimoto's thyroiditis (chronic autoimmune thyroiditis).[8][9] Hashimoto's may be associated with a goitre. It is characterized by infiltration of the thyroid gland with T lymphocytes and autoantibodies against specific thyroid antigens such as thyroid peroxidase, thyroglobulin and the TSH receptor.[8]
A more uncommon cause of hypothyroidism is estrogen dominance. It is one of the most common hormone imbalance problems in women, but is not always linked with hypothyroidism[35] There are three different types of estrogens: estrone (E1), estradiol (E2), and estriol (E3), with estradiol being the most potent of the three[35][failed verification] Women with estrogen dominance have estradiol levels of 115 pg/ml on day 3 of their cycle. However, estrogen dominance is not just about an over abundance of estradiol, but is more likely correlated with an imbalance between estradiol and progesterone. Women who have too much unopposed estrogen, which is estrogen that does not have enough counterbalancing progesterone in their bodies, commonly have unbalanced thyroid levels, in addition to excess growths within their uteri.[36]
Estradiol disrupts thyroid hormone production because high blood levels of estrogen signal the liver to increase the production of thyroid-binding globulin (TBG). This is an inhibitor protein that binds to the thyroid hormone, reducing the amount of T3 and T4 available for use by cells.[37] Without T3 and T4, the body's cellular function begins to slow down.
After women give birth, about 5% develop postpartum thyroiditis which can occur up to nine months afterwards.[38] This is characterized by a short period of hyperthyroidism followed by a period of hypothyroidism; 20–40% remain permanently hypothyroid.[38]
Autoimmune thyroiditis (Hashimoto's) is associated with other immune-mediated diseases such as diabetes mellitus type 1, pernicious anemia, myasthenia gravis, celiac disease, rheumatoid arthritis and systemic lupus erythematosus.[8] It may occur as part of autoimmune polyendocrine syndrome (type 1 and type 2).[8]
Iatrogenic hypothyroidism can be surgical (a result of thyroidectomy, usually for thyroid nodules or cancer) or following radioiodine ablation (usually for Graves' disease).
| Type of hypothyroidism | Causes |
|---|---|
| Primary hypothyroidism[8] | Iodine deficiency (developing countries), autoimmune thyroiditis, subacute granulomatous thyroiditis, subacute lymphocytic thyroiditis, postpartum thyroiditis, previous thyroidectomy, acute infectious thyroiditis,[39] previous radioiodine treatment, previous external beam radiotherapy to the neck Medication: lithium-based mood stabilizers, amiodarone, interferon alpha, tyrosine kinase inhibitors such as sunitinib |
| Central hypothyroidism[10] | Lesions compressing the pituitary (pituitary adenoma, craniopharyngioma, meningioma, glioma, Rathke's cleft cyst, metastasis, empty sella, aneurysm of the internal carotid artery), surgery or radiation to the pituitary, drugs, injury, vascular disorders (pituitary apoplexy, Sheehan syndrome, subarachnoid hemorrhage), autoimmune diseases (lymphocytic hypophysitis, polyglandular disorders), infiltrative diseases (iron overload due to hemochromatosis or thalassemia, neurosarcoidosis, Langerhans cell histiocytosis), particular inherited congenital disorders, and infections (tuberculosis, mycoses, syphilis) |
| Congenital hypothyroidism[40] | Thyroid dysgenesis (75%), thyroid dyshormonogenesis (20%), maternal antibody or radioiodine transfer Syndromes: mutations (in GNAS complex locus, PAX8, TTF-1/NKX2-1, TTF-2/FOXE1), Pendred's syndrome (associated with sensorineural hearing loss) Transiently: due to maternal iodine deficiency or excess, anti-TSH receptor antibodies, certain congenital disorders, neonatal illness Central: pituitary dysfunction (idiopathic, septo-optic dysplasia, deficiency of PIT1, isolated TSH deficiency) |
Pathophysiology
[edit]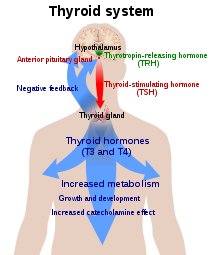
Thyroid hormone is required for the normal functioning of numerous tissues in the body. In healthy individuals, the thyroid gland predominantly secretes thyroxine (T4), which is converted into triiodothyronine (T3) in other organs by the selenium-dependent enzyme iodothyronine deiodinase.[41] Triiodothyronine binds to the thyroid hormone receptor in the nucleus of cells, where it stimulates the turning on of particular genes and the production of specific proteins.[42] Additionally, the hormone binds to integrin αvβ3 on the cell membrane, thereby stimulating the sodium–hydrogen antiporter and processes such as formation of blood vessels and cell growth.[42] In blood, almost all thyroid hormone (99.97%) are bound to plasma proteins such as thyroxine-binding globulin; only the free unbound thyroid hormone is biologically active.[8]
Electrocardiograms are abnormal in both primary overt hypothyroidism and subclinical hypothyroidism.[43] T3 and TSH are essential for regulation of cardiac electrical activity.[43] Prolonged ventricular repolarization and atrial fibrillation are often seen in hypothyroidism.[43]
The thyroid gland is the only source of thyroid hormone in the body; the process requires iodine and the amino acid tyrosine. Iodine in the bloodstream is taken up by the gland and incorporated into thyroglobulin molecules. The process is controlled by the thyroid-stimulating hormone (TSH, thyrotropin), which is secreted by the pituitary. Not enough iodine, or not enough TSH, can result in decreased production of thyroid hormones.[34]
The hypothalamic–pituitary–thyroid axis plays a key role in maintaining thyroid hormone levels within normal limits. Production of TSH by the anterior pituitary gland is stimulated in turn by thyrotropin-releasing hormone (TRH), released from the hypothalamus. Production of TSH and TRH is decreased by thyroxine by a negative feedback process. Not enough TRH, which is uncommon, can lead to not enough TSH and thereby to not enough thyroid hormone production.[10]
Pregnancy leads to marked changes in thyroid hormone physiology. The gland is increased in size by 10%, thyroxine production is increased by 50%, and iodine requirements are increased. Many women have normal thyroid function but have immunological evidence of thyroid autoimmunity (as evidenced by autoantibodies) or are iodine deficient, and develop evidence of hypothyroidism before or after giving birth.[44]
Diagnosis
[edit]Laboratory testing of thyroid stimulating hormone levels in the blood is considered the best initial test for hypothyroidism; a second TSH level is often obtained several weeks later for confirmation.[45] Levels may be abnormal in the context of other illnesses, and TSH testing in hospitalized people is discouraged unless thyroid dysfunction is strongly suspected[8] as the cause of the acute illness.[17] An elevated TSH level indicates that the thyroid gland is not producing enough thyroid hormone, and free T4 levels are then often obtained.[8][17][33] Measuring T3 is discouraged by the AACE in the assessment for hypothyroidism.[8] In England and Wales, the National Institute for Health and Care Excellence (NICE) recommends routine T4 testing in children, and T3 testing in both adults and children if central hypothyroidism is suspected and the TSH is low.[17] There are a number of symptom rating scales for hypothyroidism; they provide a degree of objectivity but have limited use for diagnosis.[8]
| TSH | T4 | Interpretation |
|---|---|---|
| Normal | Normal | Normal thyroid function |
| Elevated | Low | Overt hypothyroidism |
| Normal/low | Low | Central hypothyroidism |
| Elevated | Normal | Subclinical hypothyroidism |
Many cases of hypothyroidism are associated with mild elevations in creatine kinase and liver enzymes in the blood. They typically return to normal when hypothyroidism has been fully treated.[8] Levels of cholesterol, low-density lipoprotein and lipoprotein (a) can be elevated;[8] the impact of subclinical hypothyroidism on lipid parameters is less well-defined.[31]
Very severe hypothyroidism and myxedema coma are characteristically associated with low sodium levels in the blood together with elevations in antidiuretic hormone, as well as acute worsening of kidney function due to a number of causes.[19] In most causes, however, it is unclear if the relationship is causal.[46]
A diagnosis of hypothyroidism without any lumps or masses felt within the thyroid gland does not require thyroid imaging; however, if the thyroid feels abnormal, diagnostic imaging is then recommended.[45] The presence of antibodies against thyroid peroxidase (TPO) makes it more likely that thyroid nodules are caused by autoimmune thyroiditis, but if there is any doubt, a needle biopsy may be required.[8]
Central
[edit]If the TSH level is normal or low and serum free T4 levels are low, this is suggestive of central hypothyroidism (not enough TSH or TRH secretion by the pituitary gland or hypothalamus). There may be other features of hypopituitarism, such as menstrual cycle abnormalities and adrenal insufficiency. There might also be symptoms of a pituitary mass such as headaches and vision changes. Central hypothyroidism should be investigated further to determine the underlying cause.[10][45]
Overt
[edit]In overt primary hypothyroidism, TSH levels are high and T4 and T3 levels are low. Overt hypothyroidism may also be diagnosed in those who have a TSH on multiple occasions of greater than 5mIU/L, appropriate symptoms, and only a borderline low T4.[47] It may also be diagnosed in those with a TSH of greater than 10mIU/L.[47]
Subclinical
[edit]Subclinical hypothyroidism is a biochemical diagnosis characterized by an elevated serum TSH level, but with a normal serum free thyroxine level.[48][49][50] The incidence of subclinical hypothyroidism is estimated to be 3-15% and a higher incidence is seen in elderly people, females and those with lower iodine levels.[48] Subclinical hypothyroidism is most commonly caused by autoimmune thyroid diseases, especially Hashimoto's thyroiditis.[51] The presentation of subclinical hypothyroidism is variable and classic signs and symptoms of hypothyroidism may not be observed.[49] Of people with subclinical hypothyroidism, a proportion will develop overt hypothyroidism each year. In those with detectable antibodies against thyroid peroxidase (TPO), this occurs in 4.3%, while in those with no detectable antibodies, this occurs in 2.6%.[8] In addition to detectable anti-TPO antibodies, other risk factors for conversion from subclinical hypothyroidism to overt hypothyroidism include female sex or in those with higher TSH levels or lower level of normal free T4 levels.[48] Those with subclinical hypothyroidism and detectable anti-TPO antibodies who do not require treatment should have repeat thyroid function tested more frequently (e.g. every 6 months) compared with those who do not have antibodies.[45][48]
Pregnancy
[edit]During pregnancy, the thyroid gland must produce 50% more thyroid hormone to provide enough thyroid hormone for the developing fetus and the expectant mother.[29] In pregnancy, free thyroxine levels may be lower than anticipated due to increased binding to thyroid binding globulin and decreased binding to albumin. They should either be corrected for the stage of pregnancy,[44] or total thyroxine levels should be used instead for diagnosis.[8] TSH values may also be lower than normal (particularly in the first trimester) and the normal range should be adjusted for the stage of pregnancy.[8][44]
In pregnancy, subclinical hypothyroidism is defined as a TSH between 2.5 and 10 mIU/L with a normal thyroxine level, while those with TSH above 10 mIU/L are considered to be overtly hypothyroid even if the thyroxine level is normal.[44] Antibodies against TPO may be important in making decisions about treatment, and should, therefore, be determined in women with abnormal thyroid function tests.[8]
Determination of TPO antibodies may be considered as part of the assessment of recurrent miscarriage, as subtle thyroid dysfunction can be associated with pregnancy loss,[8] but this recommendation is not universal,[52] and presence of thyroid antibodies may not predict future outcome.[53]
Prevention
[edit]
Hypothyroidism may be prevented in a population by adding iodine to commonly used foods. This public health measure has eliminated endemic childhood hypothyroidism in countries where it was once common. In addition to promoting the consumption of iodine-rich foods such as dairy and fish, many countries with moderate iodine deficiency have implemented universal salt iodization.[54] Encouraged by the World Health Organization,[55] 70% of the world's population across 130 countries are receiving iodized salt. In some countries, iodized salt is added to bread.[54] Despite this, iodine deficiency has reappeared in some Western countries as a result of attempts to reduce salt intake.[54]
Pregnant and breastfeeding women, who require 66% more daily iodine than non-pregnant women, may still not be getting enough iodine.[54][56] The World Health Organization recommends a daily intake of 250 μg for pregnant and breastfeeding women.[57] As many women will not achieve this from dietary sources alone, the American Thyroid Association recommends a 150 μg daily supplement by mouth.[44][58]
Screening
[edit]Screening for hypothyroidism is performed in the newborn period in many countries, generally using TSH. This has led to the early identification of many cases and thus the prevention of developmental delay.[59] It is the most widely used newborn screening test worldwide.[60] While TSH-based screening will identify the most common causes, the addition of T4 testing is required to pick up the rarer central causes of neonatal hypothyroidism.[30] If T4 determination is included in the screening done at birth, this will identify cases of congenital hypothyroidism of central origin in 1:16,000 to 1:160,000 children. Considering that these children usually have other pituitary hormone deficiencies, early identification of these cases may prevent complications.[10]
In adults, widespread screening of the general population is a matter of debate. Some organizations (such as the United States Preventive Services Task Force) state that evidence is insufficient to support routine screening,[61] while others (such as the American Thyroid Association) recommend either intermittent testing above a certain age in all sexes or only in women.[8] Targeted screening may be appropriate in a number of situations where hypothyroidism is common: other autoimmune diseases, a strong family history of thyroid disease, those who have received radioiodine or other radiation therapy to the neck, those who have previously undergone thyroid surgery, those with an abnormal thyroid examination, those with psychiatric disorders, people taking amiodarone or lithium, and those with a number of health conditions (such as certain heart and skin conditions).[8] Yearly thyroid function tests are recommended in people with Down syndrome, as they are at higher risk of thyroid disease.[62] Guidelines for England and Wales from the National Institute for Health and Care Excellence (NICE) recommend testing for thyroid disease in people with type 1 diabetes and new-onset atrial fibrillation, and suggests testing in those with depression or unexplained anxiety (all ages), in children with abnormal growth, or unexplained change in behaviour or school performance.[17] On diagnosis of autoimmune thyroid disease, NICE also recommends screening for celiac disease.[63]
Management
[edit]Hormone replacement
[edit]Most people with hypothyroidism symptoms and confirmed thyroxine deficiency are treated with a synthetic long-acting form of thyroxine, known as levothyroxine (L-thyroxine).[8][16] In young and otherwise healthy people with overt hypothyroidism, a full replacement dose (adjusted by weight) can be started immediately; in the elderly and people with heart disease a lower starting dose is recommended to prevent over supplementation and risk of complications.[8][34][17] Lower doses may be sufficient in those with subclinical hypothyroidism, while people with central hypothyroidism may require a higher than average dose.[8]
Blood free thyroxine and TSH levels are monitored to help determine whether the dose is adequate. This is done 4–8 weeks after the start of treatment or a change in levothyroxine dose. Once the adequate replacement dose has been established, the tests can be repeated after 6 and then 12 months, unless there is a change in symptoms.[8] Normalization of TSH does not mean that other abnormalities associated with hypothyroidism improve entirely, such as elevated cholesterol levels.[64]
In people with central/secondary hypothyroidism, TSH is not a reliable marker of hormone replacement and decisions are based mainly on the free T4 level.[8][10] Levothyroxine is best taken 30–60 minutes before breakfast, or four hours after food,[8] as certain substances such as food and calcium can inhibit the absorption of levothyroxine.[65] There is no direct way of increasing thyroid hormone secretion by the thyroid gland.[16]
Liothyronine
[edit]Treatment with liothyronine (synthetic T3) alone has not received enough study to make a recommendation as to its use; due to its shorter half-life it would need to be taken more often than levothyroxine.[8]
Adding liothyronine to levothyroxine has been suggested as a measure to provide better symptom control, but this has not been confirmed by studies.[9][16][66] In 2007, the British Thyroid Association stated that combined T4 and T3 therapy carried a higher rate of side effects and no benefit over T4 alone.[16][67] Similarly, American guidelines discourage combination therapy due to a lack of evidence, although they acknowledge that some people feel better when receiving combination treatment.[8] Guidelines by NICE for England and Wales discourage liothyronine.[17]
People with hypothyroidism who do not feel well despite optimal levothyroxine dosing may request adjunctive treatment with liothyronine. A 2012 guideline from the European Thyroid Association recommends that support should be offered with regards to the chronic nature of the disease and that other causes of the symptoms should be excluded. Addition of liothyronine should be regarded as experimental, initially only for a trial period of 3 months, and in a set ratio to the current dose of levothyroxine.[68] The guideline explicitly aims to enhance the safety of this approach and to counter its indiscriminate use.[68]
Desiccated animal thyroid
[edit]Desiccated thyroid extract is an animal-based thyroid gland extract,[16] most commonly from pigs. It is a combination therapy, containing forms of T4 and T3.[16] It also contains calcitonin (a hormone produced in the thyroid gland involved in the regulation of calcium levels), T1 and T2; these are not present in synthetic hormone medication.[69] This extract was once a mainstream hypothyroidism treatment, but its use today is unsupported by evidence;[9][16] British Thyroid Association and American professional guidelines discourage its use,[8][67] as does NICE.[17]
Subclinical hypothyroidism
[edit]There is no evidence of a benefit from treating subclinical hypothyroidism in those who are not pregnant, and there are potential risks of overtreatment.[70] Untreated subclinical hypothyroidism may be associated with a modest increase in the risk of coronary artery disease when the TSH is over 10 mIU/L.[70][71] There may be an increased risk for cardiovascular death.[72] A 2007 review found no benefit of thyroid hormone replacement except for "some parameters of lipid profiles and left ventricular function".[73] There is no association between subclinical hypothyroidism and an increased risk of bone fractures,[74] nor is there a link with cognitive decline.[75]
American guidelines recommend that treatment should be considered in people with symptoms of hypothyroidism, detectable antibodies against thyroid peroxidase, a history of heart disease or are at an increased risk for heart disease, if the TSH is elevated but below 10 mIU/L.[8] American guidelines further recommend universal treatment (independent of risk factors) in those with TSH levels that are markedly elevated; above 10 mIU/L because of an increased risk of heart failure or death due to cardiovascular disease.[8][48] NICE recommends that those with a TSH above 10 mIU/L should be treated in the same way as overt hypothyroidism. Those with an elevated TSH but below 10 mIU/L who have symptoms suggestive of hypothyroidism should have a trial of treatment but with the aim to stopping this if the symptoms persist despite normalisation of the TSH.[17]
Myxedema coma
[edit]Myxedema coma or severe decompensated hypothyroidism usually requires admission to the intensive care, close observation and treatment of abnormalities in breathing, temperature control, blood pressure, and sodium levels. Mechanical ventilation may be required, as well as fluid replacement, vasopressor agents, careful rewarming, and corticosteroids (for possible adrenal insufficiency which can occur together with hypothyroidism). Careful correction of low sodium levels may be achieved with hypertonic saline solutions or vasopressin receptor antagonists.[19] For rapid treatment of the hypothyroidism, levothyroxine or liothyronine may be administered intravenously, particularly if the level of consciousness is too low to be able to safely swallow medication.[19] While administration through a nasogastric tube is possible, this may be unsafe and is discouraged.[19]
Pregnancy
[edit]In women with known hypothyroidism who become pregnant, it is recommended that serum TSH levels are closely monitored. Levothyroxine should be used to keep TSH levels within the normal range for that trimester. The first trimester normal range is below 2.5 mIU/L and the second and third trimesters normal range is below 3.0 mIU/L.[16][44] Treatment should be guided by total (rather than free) thyroxine or by the free T4 index. Similarly to TSH, the thyroxine results should be interpreted according to the appropriate reference range for that stage of pregnancy.[8] The levothyroxine dose often needs to be increased after pregnancy is confirmed,[8][34][44] although this is based on limited evidence and some recommend that it is not always required; decisions may need to based on TSH levels.[76]
Women with anti-TPO antibodies who are trying to become pregnant (naturally or by assisted means) may require thyroid hormone supplementation even if the TSH level is normal. This is particularly true if they have had previous miscarriages or have been hypothyroid in the past.[8] Supplementary levothyroxine may reduce the risk of preterm birth and possibly miscarriage.[77] The recommendation is stronger in pregnant women with subclinical hypothyroidism (defined as TSH 2.5–10 mIU/L) who are anti-TPO positive, in view of the risk of overt hypothyroidism. If a decision is made not to treat, close monitoring of the thyroid function (every 4 weeks in the first 20 weeks of pregnancy) is recommended.[8][44] If anti-TPO is not positive, treatment for subclinical hypothyroidism is not currently recommended.[44] It has been suggested that many of the aforementioned recommendations could lead to unnecessary treatment, in the sense that the TSH cutoff levels may be too restrictive in some ethnic groups; there may be little benefit from treatment of subclinical hypothyroidism in certain cases.[76]
Alternative medicine
[edit]The effectiveness and safety of using Chinese herbal medicines to treat hypothyroidism is not known.[78]
Epidemiology
[edit]Hypothyroidism is the most frequent endocrine disorder.[43] Worldwide about one billion people are estimated to be iodine deficient; however, it is unknown how often this results in hypothyroidism.[11] In large population-based studies in Western countries with sufficient dietary iodine, 0.3–0.4% of the population have overt hypothyroidism. A larger proportion, 4.3–8.5%, have subclinical hypothyroidism.[8] Undiagnosed hypothyroidism is estimated to affect about 4–7% of community-derived populations in the US and Europe.[79] Of people with subclinical hypothyroidism, 80% have a TSH level below the 10 mIU/L mark regarded as the threshold for treatment.[50] Children with subclinical hypothyroidism often return to normal thyroid function, and a small proportion develops overt hypothyroidism (as predicted by evolving antibody and TSH levels, the presence of celiac disease, and the presence of a goitre).[80]
Women are more likely to develop hypothyroidism than men. In population-based studies, women were seven times more likely than men to have TSH levels above 10 mU/L.[8] 2–4% of people with subclinical hypothyroidism will progress to overt hypothyroidism each year. The risk is higher in those with antibodies against thyroid peroxidase.[8][50] Subclinical hypothyroidism is estimated to affect approximately 2% of children; in adults, subclinical hypothyroidism is more common in the elderly, and in White people.[49] There is a much higher rate of thyroid disorders, the most common of which is hypothyroidism, in individuals with Down syndrome[30][62] and Turner syndrome.[30]
Very severe hypothyroidism and myxedema coma are rare, with it estimated to occur in 0.22 per million people a year.[19] The majority of cases occur in women over 60 years of age, although it may happen in all age groups.[19]
Most hypothyroidism is primary in nature. Central/secondary hypothyroidism affects 1:20,000 to 1:80,000 of the population, or about one out of every thousand people with hypothyroidism.[10]
History
[edit]In 1811, Bernard Courtois discovered iodine was present in seaweed, and iodine intake was linked with goitre size in 1820 by Jean-Francois Coindet.[81] Gaspard Adolphe Chatin proposed in 1852 that endemic goitre was the result of not enough iodine intake, and Eugen Baumann demonstrated iodine in thyroid tissue in 1896.[81]
The first cases of myxedema were recognized in the mid-19th century (the 1870s), but its connection to the thyroid was not discovered until the 1880s when myxedema was observed in people following the removal of the thyroid gland (thyroidectomy).[82] The link was further confirmed in the late 19th century when people and animals who had had their thyroid removed showed improvement in symptoms with transplantation of animal thyroid tissue.[9] The severity of myxedema, and its associated risk of mortality and complications, created interest in discovering effective treatments for hypothyroidism.[82] Transplantation of thyroid tissue demonstrated some efficacy, but recurrences of hypothyroidism was relatively common, and sometimes required multiple repeat transplantations of thyroid tissue.[82]
In 1891, the English physician George Redmayne Murray introduced subcutaneously injected sheep thyroid extract,[83] followed shortly after by an oral formulation.[9][84] Purified thyroxine was introduced in 1914 and in the 1930s synthetic thyroxine became available, although desiccated animal thyroid extract remained widely used. Liothyronine was identified in 1952.[9]
Early attempts at titrating therapy for hypothyroidism proved difficult. After hypothyroidism was found to cause a lower basal metabolic rate, this was used as a marker to guide adjustments in therapy in the early 20th century (around 1915).[82] However, a low basal metabolic rate was known to be non-specific, also present in malnutrition.[82] The first laboratory test to be helpful in assessing thyroid status was the serum protein-bound iodine, which came into use around the 1950s.
In 1971, the thyroid stimulating hormone (TSH) radioimmunoassay was developed, which was the most specific marker for assessing thyroid status in patients.[82] Many people who were being treated based on basal metabolic rate, minimizing hypothyroid symptoms, or based on serum protein-bound iodine, were found to have excessive thyroid hormone.[82] The following year, in 1972, a T3 radioimmunoassay was developed, and in 1974, a T4 radioimmunoassay was developed.[82]
Other animals
[edit]
In veterinary practice, dogs are the species most commonly affected by hypothyroidism. The majority of cases occur as a result of primary hypothyroidism, of which two types are recognized: lymphocytic thyroiditis, which is probably immune-driven and leads to destruction and fibrosis of the thyroid gland, and idiopathic atrophy, which leads to the gradual replacement of the gland by fatty tissue.[13][85] There is often lethargy, cold intolerance, exercise intolerance, and weight gain. Furthermore, skin changes and fertility problems are seen in dogs with hypothyroidism, as well as a number of other symptoms.[85] The signs of myxedema can be seen in dogs, with prominence of skin folds on the forehead, and cases of myxedema coma are encountered.[13] The diagnosis can be confirmed by blood test, as the clinical impression alone may lead to overdiagnosis.[13][85] Lymphocytic thyroiditis is associated with detectable antibodies against thyroglobulin, although they typically become undetectable in advanced disease.[85] Treatment is with thyroid hormone replacement.[13]
Other species that are less commonly affected include cats and horses, as well as other large domestic animals. In cats, hypothyroidism is usually the result of other medical treatment such as surgery or radiation. In young horses, congenital hypothyroidism has been reported predominantly in Western Canada and has been linked with the mother's diet.[13]
References
[edit]- ^ "hypothyroidism". Dictionary.com Unabridged (Online). n.d.
- ^ "hypothyroidism - definition of hypothyroidism in English from the Oxford dictionary". OxfordDictionaries.com. Archived from the original on March 11, 2013. Retrieved 2016-01-20.
- ^ a b c d e f g h i j k l m n o p q "Hypothyroidism". National Institute of Diabetes and Digestive and Kidney Diseases. March 2013. Archived from the original on 5 March 2016. Retrieved 5 March 2016.
- ^ "Psychological symptoms and thyroid disorders". British Thyroid Foundation. 11 September 2019.
- ^ a b Preedy V (2009). Comprehensive Handbook of Iodine Nutritional, Biochemical, Pathological and Therapeutic Aspects. Burlington: Elsevier. p. 616. ISBN 9780080920863.
- ^ Ferri FF (2010). Ferri's differential diagnosis : a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed.). Philadelphia, PA: Elsevier/Mosby. p. Chapter H. ISBN 978-0323076999.
- ^ a b Syed S (April 2015). "Iodine and the "near" eradication of cretinism". Pediatrics. 135 (4): 594–6. doi:10.1542/peds.2014-3718. PMID 25825529. S2CID 27647943.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA (December 2012). "Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association". Thyroid. 22 (12): 1200–35. doi:10.1089/thy.2012.0205. PMID 22954017.
- ^ a b c d e f g h Chakera AJ, Pearce SH, Vaidya B (2012). "Treatment for primary hypothyroidism: current approaches and future possibilities". Drug Design, Development and Therapy (Review). 6: 1–11. doi:10.2147/DDDT.S12894. PMC 3267517. PMID 22291465.
- ^ a b c d e f g h Persani L (September 2012). "Clinical review: Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges". The Journal of Clinical Endocrinology and Metabolism (Review). 97 (9): 3068–78. doi:10.1210/jc.2012-1616. PMID 22851492.
- ^ a b Cooper, DS, Braverman LE, eds. (2012-07-12). Werner & Ingbar's the thyroid : a fundamental and clinical text (10th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health. p. 552. ISBN 978-1451120639. Archived from the original on 2016-05-20.
- ^ Hytting J (2024-12-23). "Prevalence of abnormal thyroid hormone levels in acute new-onset atrial fibrillation". Frontiers in Cardiovascular Medicine. 11.
- ^ a b c d e f "Hypothyroidism". Merck Veterinary Manual, 10th edition (online version). 2012. Archived from the original on 2012-08-23. Retrieved 2013-12-25.
- ^ Mosby's Medical Dictionary (9 ed.). Elsevier Health Sciences. 2013. p. 887. ISBN 9780323112581. Archived from the original on 2016-03-07.
- ^ a b c d e Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J (2011). "341: disorders of the thyroid gland". Harrison's principles of internal medicine (18th ed.). New York: McGraw-Hill. ISBN 978-0071748896.
- ^ a b c d e f g h i Khandelwal D, Tandon N (January 2012). "Overt and subclinical hypothyroidism: who to treat and how". Drugs (Review). 72 (1): 17–33. doi:10.2165/11598070-000000000-00000. PMID 22191793. S2CID 207301404.
- ^ a b c d e f g h i "Thyroid disease: assessment and management". www.nice.org.uk. National Institute for Health and Care Excellence. 20 November 2019. Retrieved 9 March 2021.
- ^ Ebert EC (July 2010). "The thyroid and the gut". Journal of Clinical Gastroenterology. 44 (6): 402–6. doi:10.1097/MCG.0b013e3181d6bc3e. PMID 20351569. S2CID 23210397.
- ^ a b c d e f g Klubo-Gwiezdzinska J, Wartofsky L (March 2012). "Thyroid emergencies". The Medical Clinics of North America. 96 (2): 385–403. doi:10.1016/j.mcna.2012.01.015. PMID 22443982.
- ^ a b "Thyroid disease in Women". Office on Women's Health, U.S. Department of Health and Human Services. 1 February 2017. Archived from the original on 12 July 2017. Retrieved 20 July 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Postpartum Thyroiditis" (PDF). American Thyroid Association. 2014. Retrieved 20 July 2017.
- ^ a b Wang B, Xu Y, Zhang M, Zhang J, Hou X, Li J, Cai Y, Sun Z, Ban Y, Wang W (2020-12-01). "Oral and intestinal microbial features in pregnant women with hypothyroidism and their correlations with pregnancy outcomes". American Journal of Physiology. Endocrinology and Metabolism. 319 (6): E1044 – E1052. doi:10.1152/ajpendo.00234.2020. ISSN 0193-1849. PMID 33017219. S2CID 222164879.
- ^ Vermiglio F, Lo Presti VP, Moleti M, Sidoti M, Tortorella G, Scaffidi G, Castagna MG, Mattina F, Violi MA, Crisà A, Artemisia A (2004-12-01). "Attention Deficit and Hyperactivity Disorders in the Offspring of Mothers Exposed to Mild-Moderate Iodine Deficiency: A Possible Novel Iodine Deficiency Disorder in Developed Countries". The Journal of Clinical Endocrinology & Metabolism. 89 (12): 6054–6060. doi:10.1210/jc.2004-0571. ISSN 0021-972X. PMID 15579758.
- ^ Moog N, Entringer S, Heim C, Wadhwa P, Kathmann N, Buss C (2017-02-07). "Influence of maternal thyroid hormones during gestation on fetal brain development". Neuroscience. 342: 68–100. doi:10.1016/j.neuroscience.2015.09.070. PMC 4819012. PMID 26434624.
- ^ Hollowell JG, Garbe PL, Miller DT (1999-12-23). "Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child". The New England Journal of Medicine. 341 (26): 2016–2017. ISSN 0028-4793. PMID 10617396.
- ^ a b Vissenberg R, van den Boogaard E, van Wely M, van der Post JA, Fliers E, Bisschop PH, Goddijn M (July 2012). "Treatment of thyroid disorders before conception and in early pregnancy: a systematic review". Human Reproduction Update (Review). 18 (4): 360–73. doi:10.1093/humupd/dms007. PMID 22431565.
- ^ Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, Xu YH, Tao FB (2011-10-01). "Maternal Thyroid Function in the First Twenty Weeks of Pregnancy and Subsequent Fetal and Infant Development: A Prospective Population-Based Cohort Study in China". The Journal of Clinical Endocrinology & Metabolism. 96 (10): 3234–3241. doi:10.1210/jc.2011-0274. ISSN 0021-972X. PMID 21832110.
- ^ Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O (2004-07-09). "Overt and Subclinical Hypothyroidism Complicating Pregnancy". Thyroid. 12 (1): 63–68. doi:10.1089/105072502753451986. ISSN 1050-7256. PMID 11838732.
- ^ a b Negro R, Stagnaro-Green A (October 2014). "Diagnosis and management of subclinical hypothyroidism in pregnancy". BMJ. 349 (10): g4929. doi:10.1136/bmj.g4929. PMID 25288580. S2CID 21104809.
- ^ a b c d e f g h Counts D, Varma SK (July 2009). "Hypothyroidism in children". Pediatrics in Review. 30 (7): 251–8. doi:10.1542/pir.30-7-251. PMID 19570923. S2CID 29460139.
- ^ a b Pearce EN (February 2012). "Update in lipid alterations in subclinical hypothyroidism". The Journal of Clinical Endocrinology and Metabolism. 97 (2): 326–33. doi:10.1210/jc.2011-2532. PMID 22205712.
- ^ Chandna S, Bathla M (July 2011). "Oral manifestations of thyroid disorders and its management". Indian Journal of Endocrinology and Metabolism. 15 (Suppl 2): S113–6. doi:10.4103/2230-8210.83343. PMC 3169868. PMID 21966646.
- ^ a b Brown RS (2013). "Autoimmune thyroiditis in childhood". Journal of Clinical Research in Pediatric Endocrinology (Review). 5 Suppl 1 (4): 45–9. doi:10.4274/jcrpe.855. PMC 3608006. PMID 23154164.
- ^ a b c d Gaitonde DY, Rowley KD, Sweeney LB (August 2012). "Hypothyroidism: an update". American Family Physician. 86 (3): 244–51. PMID 22962987.[permanent dead link]
- ^ a b "Hormonal Imbalance: Causes, Symptoms & Treatment". Cleveland Clinic. Retrieved 2023-03-22.
- ^ "Friends or Foes? Estrogen, Progesterone & Thyroid Hormones". Dr. Laura Neville. 15 March 2022. Retrieved 2023-03-22.
- ^ Pirahanchi Y, Toro F, Jialal I (2023), "Physiology, Thyroid Stimulating Hormone", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29763025, retrieved 2023-03-22
- ^ a b Stagnaro-Green A (February 2012). "Approach to the patient with postpartum thyroiditis". The Journal of Clinical Endocrinology and Metabolism (Review). 97 (2): 334–42. doi:10.1210/jc.2011-2576. PMID 22312089.
- ^ Shrestha ST, Hennessey J (December 2015). Acute and Subacute, and Riedel's Thyroiditis. MDText.com, Inc. PMID 25905408.
- ^ Donaldson M, Jones J (2013). "Optimising outcome in congenital hypothyroidism; current opinions on best practice in initial assessment and subsequent management". Journal of Clinical Research in Pediatric Endocrinology (Review). 5 Suppl 1 (4): 13–22. doi:10.4274/jcrpe.849. PMC 3608009. PMID 23154163.
- ^ Maia AL, Goemann IM, Meyer EL, Wajner SM (June 2011). "Deiodinases: the balance of thyroid hormone: type 1 iodothyronine deiodinase in human physiology and disease". The Journal of Endocrinology. 209 (3): 283–97. doi:10.1530/JOE-10-0481. PMID 21415143.
- ^ a b Cheng SY, Leonard JL, Davis PJ (April 2010). "Molecular aspects of thyroid hormone actions". Endocrine Reviews. 31 (2): 139–70. doi:10.1210/er.2009-0007. PMC 2852208. PMID 20051527.
- ^ a b c d Casis O, Echeazarra L, Gallego M (2024). "Deciphering the roles of triiodothyronine (T3) and thyroid-stimulating hormone (TSH) on cardiac electrical remodeling in clinical and experimental hypothyroidism". Journal of Physiology and Biochemistry. 80 (1): 1–9. doi:10.1007/s13105-023-01000-z. PMC 10808292. PMID 38019451.
- ^ a b c d e f g h i Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W (October 2011). "Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum". Thyroid. 21 (10): 1081–125. doi:10.1089/thy.2011.0087. PMC 3472679. PMID 21787128.
- ^ a b c d So M, MacIsaac RJ, Grossmann M (August 2012). "Hypothyroidism" (PDF). Australian Family Physician. 41 (8): 556–62. PMID 23145394.
- ^ Pantalone KM, Hatipoglu BA (December 2014). "Hyponatremia and the Thyroid: Causality or Association?". Journal of Clinical Medicine. 4 (1): 32–6. doi:10.3390/jcm4010032. PMC 4470237. PMID 26237016.
- ^ a b Dons RF, Wians FH Jr (2009). Endocrine and metabolic disorders clinical lab testing manual (4th ed.). Boca Raton: CRC Press. p. 10. ISBN 9781420079364.
- ^ a b c d e Peeters RP (29 June 2017). "Subclinical Hypothyroidism". New England Journal of Medicine. 376 (26): 2556–2565. doi:10.1056/NEJMcp1611144. PMID 28657873. S2CID 56184355.
- ^ a b c Bona G, Prodam F, Monzani A (2013). "Subclinical hypothyroidism in children: natural history and when to treat". Journal of Clinical Research in Pediatric Endocrinology (Review). 5 Suppl 1 (4): 23–8. doi:10.4274/jcrpe.851. PMC 3608012. PMID 23154159.
- ^ a b c Fatourechi V (2009). "Subclinical hypothyroidism: an update for primary care physicians". Mayo Clinic Proceedings (Review). 84 (1): 65–71. doi:10.4065/84.1.65. PMC 2664572. PMID 19121255.
- ^ Baumgartner C, Blum MR, Rodondi N (December 2014). "Subclinical hypothyroidism: summary of evidence in 2014". Swiss Medical Weekly (Review). 144: w14058. doi:10.4414/smw.2014.14058. PMID 25536449.
- ^ Practice Committee of the American Society for Reproductive Medicine (November 2012). "Evaluation and treatment of recurrent pregnancy loss: a committee opinion". Fertility and Sterility. 98 (5): 1103–11. doi:10.1016/j.fertnstert.2012.06.048. PMID 22835448. S2CID 30527688.
- ^ "Recurrent Miscarriage, Investigation and Treatment of Couples". Royal College of Obstetricians & Gynaecologists.
- ^ a b c d Charlton K, Skeaff S (November 2011). "Iodine fortification: why, when, what, how, and who?". Current Opinion in Clinical Nutrition and Metabolic Care. 14 (6): 618–24. doi:10.1097/MCO.0b013e32834b2b30. PMID 21892078. S2CID 22906831.
- ^ World Health Organization, UNICEF, ICCIDD (2008). Assessment of iodine deficiency disorders and monitoring their elimination (PDF) (3rd ed.). Geneva: World Health Organization. ISBN 9789241595827. Archived (PDF) from the original on 2013-12-28.
- ^ e-Library of Evidence for Nutrition Actions (eLENA) (2014). "Iodine supplementation during pregnancy". World Health Organization. Archived from the original on 6 March 2014. Retrieved 5 March 2014.
- ^ "Reaching Optimal Iodine Nutrition in Pregnant and Lactating Women and Young Children" (PDF). Joint Statement by the World Health Organization and United Nations Children's Fund. World Health Organization. 2007. Archived from the original (PDF) on 6 March 2014. Retrieved 5 March 2014.
- ^ Becker DV, Braverman LE, Delange F, Dunn JT, Franklyn JA, Hollowell JG, Lamm SH, Mitchell ML, Pearce E, Robbins J, Rovet JF (October 2006). "Iodine supplementation for pregnancy and lactation-United States and Canada: recommendations of the American Thyroid Association". Thyroid. 16 (10): 949–51. doi:10.1089/thy.2006.16.949. PMID 17042677. S2CID 28515565.
- ^ Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, Varma SK (June 2006). "Update of newborn screening and therapy for congenital hypothyroidism". Pediatrics. 117 (6): 2290–303. doi:10.1542/peds.2006-0915. PMID 16740880. S2CID 1068578.
- ^ Pollitt RJ (June 2009). "Newborn blood spot screening: new opportunities, old problems". Journal of Inherited Metabolic Disease. 32 (3): 395–9. doi:10.1007/s10545-009-9962-0. PMID 19412659. S2CID 41563580.
- ^ LeFevre ML (May 2015). "Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement". Annals of Internal Medicine. 162 (9): 641–50. doi:10.7326/M15-0483. PMID 25798805. S2CID 262490923.
- ^ a b Malt EA, Dahl RC, Haugsand TM, Ulvestad IH, Emilsen NM, Hansen B, Cardenas YE, Skøld RO, Thorsen AT, Davidsen EM (February 2013). "Health and disease in adults with Down syndrome". Tidsskrift for den Norske Laegeforening. 133 (3): 290–4. doi:10.4045/tidsskr.12.0390. PMID 23381164.
- ^ "Coeliac disease: recognition, assessment and management". www.nice.org.uk. National Institute for Health and Care Excellence. 2 September 2015. Retrieved 9 March 2021.
- ^ McAninch EA, Rajan KB, Miller CH, Bianco AC (1 December 2018). "Systemic Thyroid Hormone Status During Levothyroxine Therapy In Hypothyroidism: A Systematic Review and Meta-Analysis". The Journal of Clinical Endocrinology & Metabolism. 103 (12): 4533–4542. doi:10.1210/jc.2018-01361. PMC 6226605. PMID 30124904.
- ^ Cascorbi I (August 2012). "Drug interactions--principles, examples and clinical consequences". Deutsches Ärzteblatt International (Review). 109 (33–34): 546–55, quiz 556. doi:10.3238/arztebl.2012.0546. PMC 3444856. PMID 23152742.
- ^ Escobar-Morreale HF, Botella-Carretero JI, Escobar del Rey F, Morreale de Escobar G (August 2005). "REVIEW: Treatment of hypothyroidism with combinations of levothyroxine plus liothyronine". The Journal of Clinical Endocrinology and Metabolism (Review). 90 (8): 4946–54. doi:10.1210/jc.2005-0184. hdl:10261/24668. PMID 15928247.
- ^ a b British Thyroid Association Executive Committee (November 2007). "Armour Thyroid(USP) and combinedthyroxine/tri-iodothyronine as thyroid hormone replacement" (PDF). British Thyroid Association. Archived from the original (PDF) on 3 December 2008. Retrieved 25 December 2013.
- ^ a b Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP (July 2012). "2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism". European Thyroid Journal. 1 (2): 55–71. doi:10.1159/000339444. PMC 3821467. PMID 24782999.
- ^ Ebling PR (2011). "ESA Position Statement on Desiccated Thyroid or Thyroid Extract" (PDF). Endocrine Society of Australia. Archived from the original (PDF) on 26 January 2014. Retrieved 13 December 2013.
- ^ a b Bekkering GE, Agoritsas T, Lytvyn L, Heen AF, Feller M, Moutzouri E, Abdulazeem H, Aertgeerts B, Beecher D, Brito JP, Farhoumand PD, Singh Ospina N, Rodondi N, van Driel M, Wallace E, Snel M, Okwen PM, Siemieniuk R, Vandvik PO, Kuijpers T, Vermandere M (14 May 2019). "Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline". BMJ. 365: l2006. doi:10.1136/bmj.l2006. PMID 31088853.
- ^ Ochs N, Auer R, Bauer DC, Nanchen D, Gussekloo J, Cornuz J, Rodondi N (June 2008). "Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality". Annals of Internal Medicine (Meta-analysis). 148 (11): 832–45. doi:10.7326/0003-4819-148-11-200806030-00225. PMID 18490668.
- ^ Müller P, Leow MK, Dietrich JW (2022). "Minor perturbations of thyroid homeostasis and major cardiovascular endpoints-Physiological mechanisms and clinical evidence". Frontiers in Cardiovascular Medicine. 9: 942971. doi:10.3389/fcvm.2022.942971. PMC 9420854. PMID 36046184.
- ^ Villar HC, Saconato H, Valente O, Atallah AN (July 2007). "Thyroid hormone replacement for subclinical hypothyroidism". The Cochrane Database of Systematic Reviews. 2009 (3): CD003419. doi:10.1002/14651858.CD003419.pub2. PMC 6610974. PMID 17636722.
- ^ Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, Wirth CD, Peeters RP, Åsvold BO, den Elzen WP, Luben RN, Imaizumi M, Bremner AP, Gogakos A, Eastell R, Kearney PM, Strotmeyer ES, Wallace ER, Hoff M, Ceresini G, Rivadeneira F, Uitterlinden AG, Stott DJ, Westendorp RG, Khaw KT, Langhammer A, Ferrucci L, Gussekloo J, Williams GR, Walsh JP, Jüni P, Aujesky D, Rodondi N (May 2015). "Subclinical thyroid dysfunction and fracture risk: a meta-analysis". JAMA. 313 (20): 2055–65. doi:10.1001/jama.2015.5161. PMC 4729304. PMID 26010634.
- ^ Rieben C, Segna D, da Costa BR, Collet TH, Chaker L, Aubert CE, Baumgartner C, Almeida OP, Hogervorst E, Trompet S, Masaki K, Mooijaart SP, Gussekloo J, Peeters RP, Bauer DC, Aujesky D, Rodondi N (December 2016). "Subclinical Thyroid Dysfunction and the Risk of Cognitive Decline: a Meta-Analysis of Prospective Cohort Studies". The Journal of Clinical Endocrinology and Metabolism. 101 (12): 4945–4954. doi:10.1210/jc.2016-2129. PMC 6287525. PMID 27689250.
- ^ a b Wiles KS, Jarvis S, Nelson-Piercy C (October 2015). "Are we overtreating subclinical hypothyroidism in pregnancy?". BMJ. 351: h4726. doi:10.1136/bmj.h4726. PMID 26459315. S2CID 32615623.
- ^ Reid SM, Middleton P, Cossich MC, Crowther CA, Bain E (May 2013). Reid SM (ed.). "Interventions for clinical and subclinical hypothyroidism pre-pregnancy and during pregnancy". The Cochrane Database of Systematic Reviews. 5 (5): CD007752. doi:10.1002/14651858.CD007752.pub3. PMC 11664309. PMID 23728666.
- ^ Ke Lq, Hu Y, Yang K, Tong N (12 February 2015). "Chinese herbal medicines for hypothyroidism". Cochrane Database of Systematic Reviews. 2015 (2): CD008779. doi:10.1002/14651858.CD008779.pub2. PMC 10625441. PMID 25914906.
- ^ Gottwald-Hostalek U, Schulte B (January 2022). "Low awareness and under-diagnosis of hypothyroidism". Current Medical Research and Opinion. 38 (1): 59–64. doi:10.1080/03007995.2021.1997258. PMID 34698615. S2CID 239888323.
- ^ Monzani A, Prodam F, Rapa A, Moia S, Agarla V, Bellone S, Bona G (January 2013). "Endocrine disorders in childhood and adolescence. Natural history of subclinical hypothyroidism in children and adolescents and potential effects of replacement therapy: a review". European Journal of Endocrinology. 168 (1): R1 – R11. doi:10.1530/EJE-12-0656. PMID 22989466.
- ^ a b Leung AM, Braverman LE, Pearce EN (November 2012). "History of U.S. iodine fortification and supplementation". Nutrients. 4 (11): 1740–6. doi:10.3390/nu4111740. PMC 3509517. PMID 23201844.
- ^ a b c d e f g h McAninch EA, Bianco AC (January 2016). "The History and Future of Treatment of Hypothyroidism". Annals of Internal Medicine. 164 (1): 50–6. doi:10.7326/M15-1799. PMC 4980994. PMID 26747302.
- ^ Murray GR (October 1891). "Note on the Treatment of Myxoedema by Hypodermic Injections of an Extract of the Thyroid Gland of a Sheep". British Medical Journal. 2 (1606): 796–7. doi:10.1136/bmj.2.1606.796. PMC 2273741. PMID 20753415.
- ^ Fox EL (October 1892). "A Case of Myxoedema Treated by Taking Extract of Thyroid by the Mouth". British Medical Journal. 2 (1661): 941. doi:10.1136/bmj.2.1661.941. PMC 2421284. PMID 20753901.
- ^ a b c d Mooney CT (May 2011). "Canine hypothyroidism: a review of aetiology and diagnosis". New Zealand Veterinary Journal. 59 (3): 105–14. doi:10.1080/00480169.2011.563729. PMID 21541883. S2CID 29535272.
External links
[edit]- "Hypothyroidism information for patients". American Thyroid Association. Retrieved 2017-03-25.
- "UK Guidelines for the use of thyroid function tests" (PDF). The Association for Clinical Biochemistry, British Thyroid Association and British Thyroid Foundation. 2006. Retrieved 2013-12-25.
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S (March 2017). "2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum". Thyroid. 27 (3): 315–389. doi:10.1089/thy.2016.0457. PMC 3472679. PMID 28056690.